Listen&Learn: Absorption lines
Posted by: Jaksyn PeacockPre-listening vocabulary
- prism: a glass object that splits white light into colours
- spectrum: the range of colours that a prism reveals
- block: to hide something from view
- particle: a tiny piece of something
- element: one of the basic chemicals found on the periodic table
- random: happening without a pattern or reason
- unique: specific to one person or thing
Listening activity
Podcast: Play in new window | Download (Duration: 1:43 — 1.6MB)
Subscribe: Apple Podcasts | Google Podcasts | RSS | More
Gapfill exercise
Comprehension questions
See answers below
- Joseph von Fraunhofer discovered that
a. passing light through a prism would create a rainbow
b. light could behave as a particle
c. there were dark lines in the colour spectrum made by the Sun - Absorption lines happen when
a. a cool gas absorbs photons
b. a hot gas releases photons
c. photons pass through empty space - Scientists can study absorption lines to find out
a. the masses of stars
b. the distances between stars
c. the chemicals present in stars
Discussion/essay questions
- Do you know any interesting facts about light? What optical illusions or “tricks of the light” have you seen?
Transcript
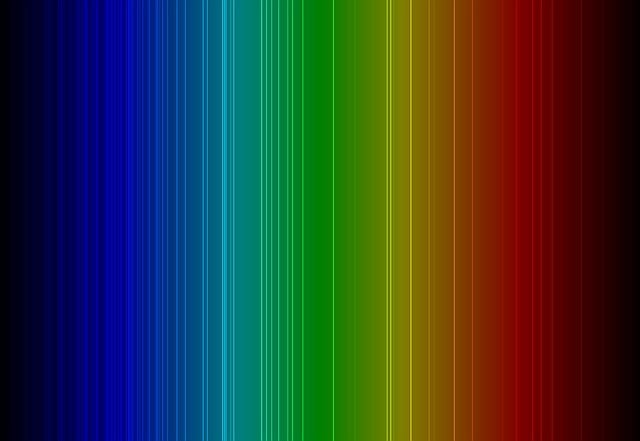
In 1814, a German physicist named Joseph von Fraunhofer passed light from the Sun through a prism to create a colour spectrum. He noticed that there were dark lines blocking out certain colours in the spectrum. Today, scientists call these lines absorption lines. Absorption lines happen when particles of light, called photons, move through a cool gas. The atoms and molecules in the gas absorb some of the photons and block certain colours of light. When an atom absorbs a photon, it shows up as a dark line in the colour spectrum. The photons that get absorbed are not random. Every element absorbs specific wavelengths of light, which means every element also has a unique pattern of absorption lines. Scientists can study these lines to find out which chemicals are present in stars and planets.
Answers to comprehension questions
1c 2a 3c
Search for more Listen&Learn stories:
Subscribe to EnglishClub Podcasts
4 comments
-
Omnia Samir says:
What a great way to learn a language with learning loads of different information about a real life which means I appreciate talking about different aspects of life
-
Diego Reyes says:
Wanderfull, this space is wanderfull!The way you take so diferents topics of cience its really nice, its to enjoy! Congratulations and thanks a lot.
-
Delva Lamarre says:
That’s very insightfull
-
Hojin Jeong says:
That’s a good podcast.